Protein mechanics and stability
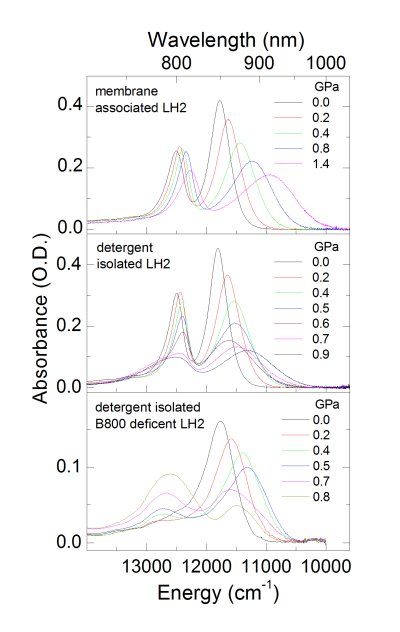
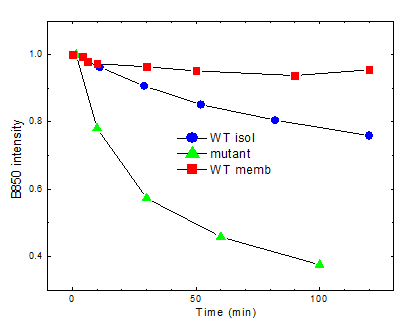
Proteins carry out many of the most important tasks in living organisms. The protein function is defined by its folded structure, while denatured conformations result in disorders. Understanding of the protein stability is thus equally important for solving fundamental problems like protein folding as well as for practical (medical) applications. Proteins can be denatured by changes of physical parameters such as temperature and pressure. In photosynthetic bacteria these proteins, called antenna and reaction centre complexes, show characteristic intense absorption bands at near-infrared spectral region due to bacteriochlorophyll cofactors confined in separate areas of the proteins. Using the non-covalently bound bacteriochlorophyll molecules as intrinsic probes to monitor local changes of the binding sites, we investigated spectral responses of the complexes to high pressures reaching 2.5 GPa. Surprising resilience of the antenna proteins to such extreme conditions is demonstrated, even when the complexes were extracted with mild detergents out from the native membranes to less protective detergent-buffer environments. Although pressurizing does induce significant alterations to the tertiary structure of the proteins, including breakage of the hydrogen bonds the bacteriochlorophyll cofactors are involved in, the effects are largely reversible. Presence of co-solvents (glycerol) and aggregation generally further stabilize the proteins. Worth noticing, however, is that genetically modified complexes tend to disintegrate more easily under high pressure than wild type complexes by releasing large parts of their bacteriochlorophyll content. Considerable variability of elastic properties of isolated complexes is also observed, which can be assigned to heterogeneous protein packing in detergent micelles. Our work draws attention to both the high-pressure spectroscopy as effective technique and to the pigmented photosynthetic complexes as useful model systems for in detail studies of the stability of integral membrane proteins.